Multi-photon microscopy to better treat diseases
- To understand fundamental physiological mechanisms and processes, as well as those of many pathologies, it is essential to observe cellular metabolic activity in real-time with subcellular resolution.
- In biology, it is common to “tag” molecules with fluorescence markers, but this is an invasive technique, as one has to “enter” the cell.
- However, there are fluorescent molecules already present in cells that can be stimulated without artificially integrating them and interfering with the living system.
- Acquiring this type of knowledge opens the way to many applications in medicine, as it allows the metabolism of cells – an indicator of their activity – to be mapped. This, for example, allows the identification of the metabolism of cancer cells, which are likely to develop many forms of cancer.
Chiara Stringari, a researcher at the Laboratory of Optics and Biosciences (LOB) at École Polytechnique, is skirting at the frontier of several different scientific disciplines. Her aim is to develop innovative microscopic observation techniques to map the metabolic activity of cells and tissues with greater precision. To do this, she uses a technique based on two-photon optical microscopy; a method using two photons projected onto a target cell, through a laser, which make a molecule in that cell react so it becomes visible by fluorescence. Correct functioning of a healthy cell is determined by activity of the molecules within it. Observing them and characterising their fluorescence lifetime with greater precision allows researchers to identify the molecular signature, and thus normal cellular function, so that they can establish medical diagnoses.
Observing cellular metabolic activity in real-time with subcellular resolution is crucial to understanding fundamental physiological mechanisms and processes as well as those of several pathologies. To do this, it is common to “tag” molecules with fluorescence markers, but this requires an invasive technique, as one has to “enter” the cell. As such, an invasive technique will always have an impact on the organism being observed, however small. Chiara Stringari, on the other hand, relies on the fluorescent molecules already present in cells – in particular NADH and FAD, which are present in all our living cells and provide information on metabolism. They can be stimulated without being artificially interfering with the living system. It is technique that is still in the pre-clinical phase. So, tests on humans have therefore not yet begun; experiments are still limited to the in vitro (on artificial organisms) and in vivo (in particular on zebrafish and mice) stages.
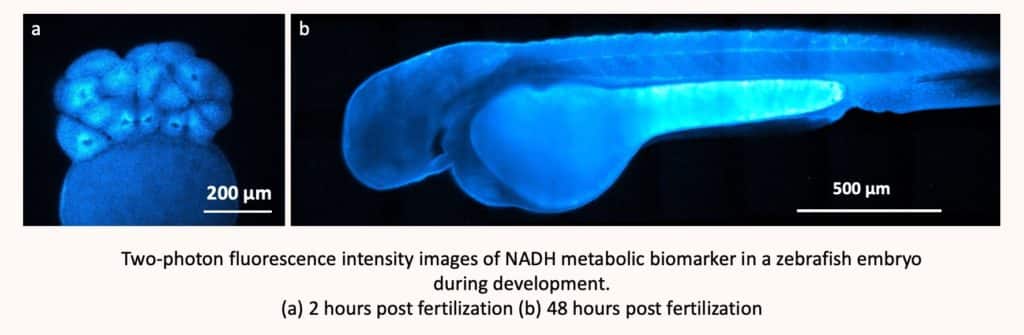
Observing the molecule without tagging it
Bypassing the ‘tagging’ stage therefore makes this technique, which is still under development, as innovative as it is useful. “It is the core of my research to develop new methods using non-linear optics,” states Chiara Stringari. It gives researchers access to new information, while using contrasts or endogenous biomarkers – that remove the need for tagging – so that this technique is as non-invasive as possible.” The ability to observe the activity of molecules, like that of any other cell in our body, as closely as possible, thus allows us to understand their development, without damaging the biological systems observed.
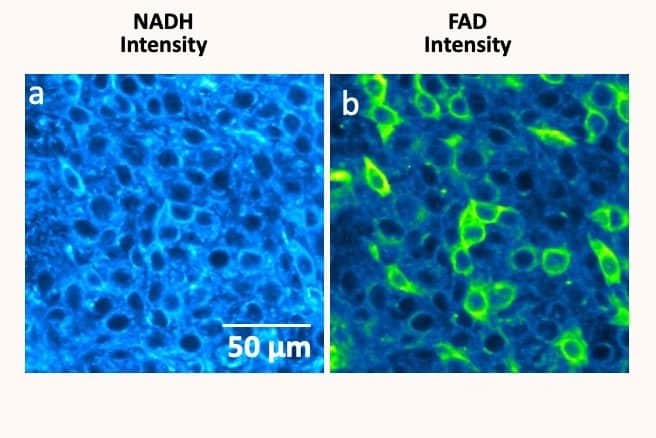
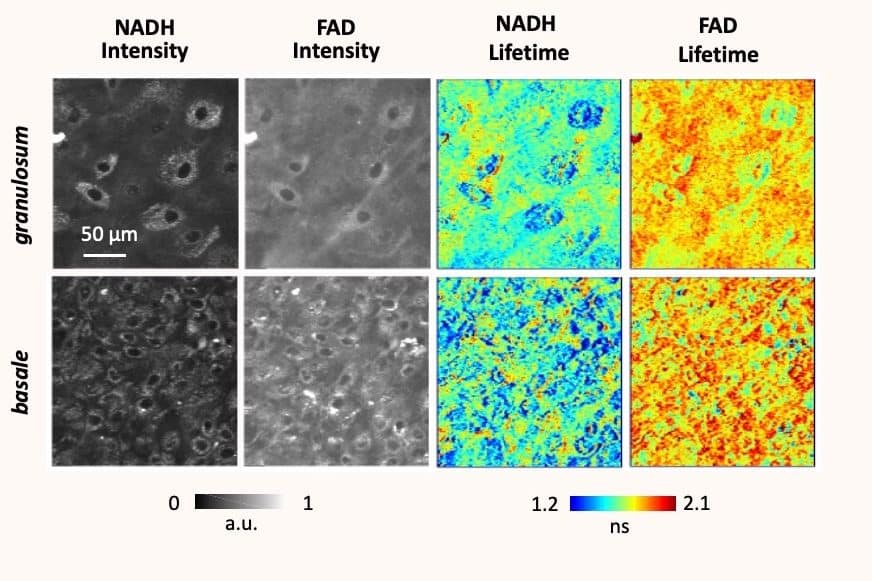
Acquiring this type of knowledge opens the way to many applications in medicine, as it allows researchers to map cellular metabolism – an indicator of activity in cells. “Especially since metabolism, which is very important for development, affects epigenetics,” she explains. “Mapping allows us to create a model and better understand the interest and role of the different connections between cells. And therefore, to understand the interactions they have with their environment.” This, in turn, allows the identification of the metabolism of cancer cells, which are likely to develop many forms of cancer. “The biomarkers used allowed us to dissociate healthy cells from cancer cells. The biomarkers used have allowed us to separate healthy cells from cancer cells, allowing us to establish phenotypic metabolic subtypes of cancer.”
Better understand how brain cells work
“The aim is not to diagnose, but this technique does provide many tools to facilitate this. In my opinion, the most important thing is to better understand how our brain cells work.”
These observations give us information about the environment of the molecules. It allows us to understand how they interact with their surroundings, and what activates them. The researchers make an experimental measurement on a sub-cellular scale (< 1 µm), in order to establish a map of the metabolism and identify the lack (or abundance) of certain molecules in our cells. Especially since “each cell has a sort of fingerprint of its metabolism”, she says.
Using another non-labelling technique – that of the third harmonic generation, which complements two-photon fluorescence – Chiara Stringari is working in particular on imaging myelin, a lipid sheath which is “very important in the connection and metabolic support of neurones,” she explains. Studying a 3D representation of myelin allows her to understand the impact that its degradation can have on the metabolism of neurones, in combination with the data obtained using the two-photon technique. “This allows us to learn more about our brains, while also providing leads for research into diagnosis, both of which are very complementary.”
Multiple sclerosis is a disease that affects myelin, causing its degradation (demyelination) and neurodegeneration. Chiara Stringari and her team have therefore undertaken research on this disease, in ex vivo conditions, with a view to establishing “the biological consequences of myelin pathology at the level of cellular metabolism and neuronal networks”. Comparing the function of distinct phases known as myelination and demyelination makes it possible to learn more about how it works, its cellular activity and, above all, its repair.
Indeed, many diseases are caused by the degradation of brain cells; these are known as neurodegenerative diseases. The studies undertaken by Chiara Stringari could help in the identification of effective therapeutic strategies. And it will allow us to go even further, by understanding how a cell initiates its degradation, we could, perhaps one day, prevent this process.