Can viruses be used to fight bacterial infections ?
- Long before Covid-19, the WHO was already warning about certain infectious agents considered "one of the most serious threats to global health".
- Responsible for 1.27 million deaths worldwide in 2019, likely to cause 10 million a year by 2050, this threat is antibiotic-resistant bacteria.
- A number of patients, infected with antibiotic-resistant bacteria, have already been saved by compassionately administered bacteriophages.
- In France, the PHAGEinLYON programme has treated several dozen patients since 2017 and recently received funding from the ANR to develop access to phage therapy.
While the main causes of death in developed countries, including France, are cancer and cardiovascular disease1, the World Health Organisation warned (well before Covid-19) that it considers certain infectious agents “one of the most serious threats to global health, food security and development”2. This threat is antibiotic-resistant bacteria, which were responsible for 1.27 million deaths worldwide in 20193 and could cause 10 million deaths annually by 20504.
Yet, it is difficult to fight against this phenomenon. Limiting and optimising the use of antibiotics can reduce the emergence of new antibiotic resistant bacteria. But the search for new antibiotics has unfortunately not been very successful, while we also need treatments capable of counteracting the antibiotic resistant bacteria that already exist… What if the solution was even older than antibiotics ?
Healing with viruses
December 1917. Félix d’Hérelle, a scientist whose biography is worth a look, published a paper in the Proceedings of the Academy of Sciences5. He described invisible microbes, capable of destroying the bacteria responsible for dysentery or typhoid fever by multiplying at their expense. He described these microbes as “bacteriophages”, i.e., literally, bacteria eaters. As virology was still in its infancy at the time, Félix d’Hérelle was not aware of it, but he had just described viruses capable of infecting and killing bacteria ! Today, they are still called bacteriophages, or phages for short.
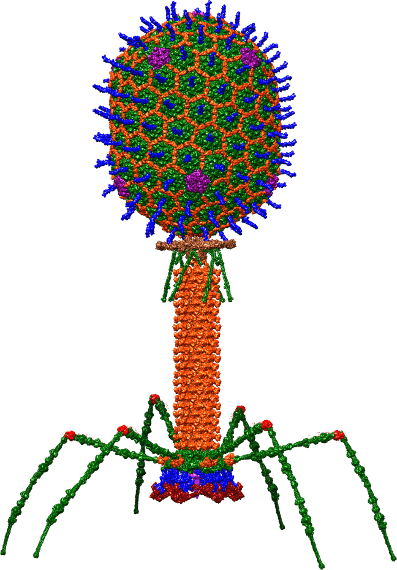
The authorship of this discovery is disputed as d’Hérelle was not the first to make this kind of observation. He was, however, the first to have the idea of using these viruses as treatments. His 1917 paper explains that phages can protect rabbits against dysentery, without any side effects, and are specific to certain strains of bacteria : the foundations of phage therapy were laid.
Indeed, since they are only a threat to bacteria, these enemies of our enemies have the right profile to become our allies. Phage therapy thus became an international craze in the 1920s ! At the time, there was no other way to fight bacterial infections. Penicillin was discovered in 1928, but it was not purified and used for medical purposes until over a decade later.
From phages to antibiotics… and back again ?
Antibiotics were inexpensive, very effective, easy to produce, store and administer. As early as the 1940s, they replaced bacteriophages, whose effectiveness was less certain, without replacing them entirely : therapeutic phages were available in France until the end of the 1980s. But Alexander Flemming, the discoverer of penicillin, was right when he warned about the bacteria’s capacity for resistance. Could phages be more effective than antibiotics in this respect ?
Unlike these inert molecules, viruses evolve spontaneously to adapt to bacterial adaptations. Their therapeutic use should reproduce the arms race classically observed between a parasite and its host instead of the dead end in which antibiotics are stuck. And there are other reasons why phages are a promising alternative !
In terms of mechanisms of action, antibiotics can be compared to bombs and bacteriophages to precision shooting : the former destroy bacteria en masse while the latter are specific to a type of bacteria. In the middle of the 20th Century, it was difficult to characterise bacterial or viral strains and the wide range of action of antibiotics was an advantage. Today, we know that our organisms are true ecosystems, containing about as many bacteria as human cells6. Bacteriophages would allow us to target only those that are pathogenic, preserving the rest of our microbiota and concentrating spontaneously at the sites of infection.
From theory to practical application
A number of patients infected with antibiotic-resistant bacteria have already been saved by compassionate administration of bacteriophages. These cures have sometimes received significant media coverage, such as that of the spouse of Steffanie Strathdee, an epidemiologist who has since become co-director of the first phage therapy research centre in the United States7.
In France, the PHAGEinLYON program8 has treated several dozen patients since 2017 and recently received funding from the ANR to expand access to phage therapy. Its results are very promising, but we are still far from being able to deploy this approach on a large scale.
Certain technical and administrative constraints remain restrictive, starting with the production of medical phages, which need to meet high-quality standards. In France, there is currently only one company9 capable of producing phages for human use. Belgium considers phages as magistral preparations, not as drugs, which facilitates their production. In any case, the question of the patentability of these biological entities is not clear-cut, which may slow down industrial investments. And there are still scientific limits to the development of phagotherapy.
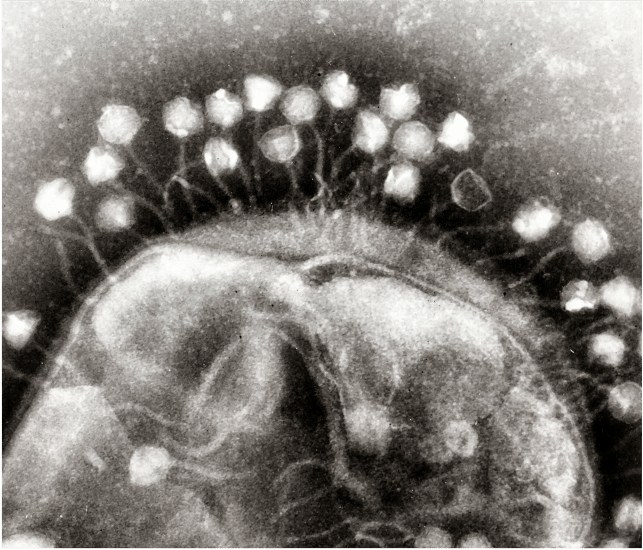
Identifying phages
For phage therapy to be effective, viruses must be identified that match the needs of each patient. It is not possible to predict which phage will be effective against a given bacterium. To find out, it is necessary to test on a case-by-case basis and hope that the effects within the patient’s microbial ecosystem will be identical to those observed in vitro. In general, patients are treated with cocktails of several potentially effective phages.
In order to target a maximum number of bacteria, we need large repertoires of phages from which to draw from. And even though there is a considerable natural diversity of phages, estimated at 108 species10, we are far from having catalogued enough of them to be able to generalise phage therapy. However, certain structures have been working on this for decades : the countries of the Soviet bloc did not have access to antibiotics during the cold war, and their use of phages is particularly developed (which generates medical tourism).
Furthermore, the efficacy of phage therapy must be properly evaluated beyond compassionate cases. A few standard clinical trials have been conducted since 2010, against infections for which phage cocktails can be standardized in a “ready-to-wear” manner. Some of these trials are promising11, but this evaluation methodology is less adapted to “customised” uses of bacteriophages.
Finally, even if it only concerns certain patients, one characteristic of phages remains limiting : some areas of the body remain inaccessible to them, such as the central nervous system or the interior of our cells.
A treatment in the making
Despite its promise, phage therapy is not (yet) a therapeutic revolution. But this new approach should be thought of in combination with the tools already at our disposal ! Interesting synergies have been observed with antibiotics, for example.
The use of certain proteins produced by bacteriophages, notably lysines, enzymes capable of degrading bacterial walls and biofilms, is also being considered as a therapeutic tool in its own right. Or to genetically modify phages to target refractory bacteria.
It’s hard to predict how bacteriophages will change our response to bacterial infections, but they seem to have the potential to meet some of our current needs, and chances are we’ll be hearing more and more about them12 !
For more about this
Related event : symposium on antibiotic resistance organized by Inserm and Institut Pasteur, June 7 : https://www.pasteur.fr/fr/journal-recherche/evenements/colloque-scientifique-antibioresistance-amr















